Dr. Vadym Zayets
v.zayets(at)gmail.com
My Research and Inventions
click here to see all content |

Dr. Vadym Zayetsv.zayets(at)gmail.com |
|
 |
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionTransport Eqs.Spin Proximity/ Spin InjectionSpin DetectionBoltzmann Eqs.Band currentScattering currentMean-free pathCurrent near InterfaceOrdinary Hall effectAnomalous Hall effect, AMR effectSpin-Orbit interactionSpin Hall effectNon-local Spin DetectionLandau -Lifshitz equationExchange interactionsp-d exchange interactionCoercive fieldPerpendicular magnetic anisotropy (PMA)Voltage- controlled magnetism (VCMA effect)All-metal transistorSpin-orbit torque (SO torque)What is a hole?spin polarizationCharge accumulationMgO-based MTJMagneto-opticsSpin vs Orbital momentWhat is the Spin?model comparisonQuestions & AnswersEB nanotechnologyReticle 11
|
Pauli paramagnetism. Pauli and Stoner models. Features and limitations. Spin and Charge TransportIn an external magnetic field, the spin polarization of conduction electrons increases and the number of spin-polarized conduction electrons becomes larger (step 1) Calulation of the spin polarization of the electron gas induced by the magnetic field The increase of the number of conduction electrons causes the increase of the magnetization of the ferromagnetic metal. This effect is called the Pauli paramagnetism.There are two reasons why the magnetization increases. (reason 1): Increases of the total spin of all conduction electrons, because of a larger number of spin-polarized conduction electrons. (reason 2): increase of the number of spin-up localized d- electrons, because of the scatterings between conduction and localized electrons.
|
Spin polarization of the electron gas is determined by a balance between spin pumping and spin relaxation |
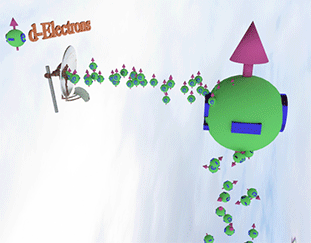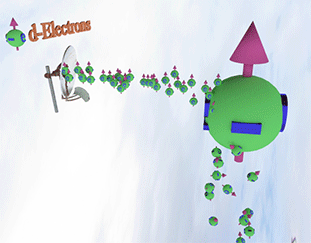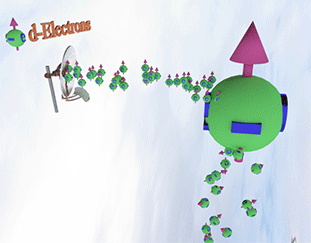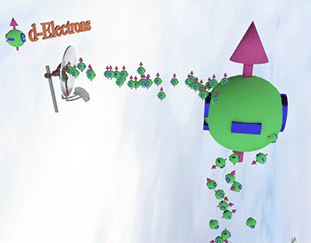 |
| arrows shows the spin-direction and the volume of balls is proportional to the number of the spin polarized electrons. The big ball shows all conduction spin-polarized electrons. |
| Spin pump source is shown as antenna. Spin pump creates electrons with spin directed in one direction. A faster spin pumping makes the spin polarization larger. The source of the spin pumping is an external magnetic field, interaction with localized d-electron and the absorption of a circular-polarized light. |
| Spin relaxation are shown as electrons falling dawn. The spin relaxation is the electron conversion from group of spin-polarized electrons (spin aligned in one direction) to group of the spin-unpolarized electrons (spin is not aligned). A larger the spin relaxation makes the spin polarization smaller. |
| click on image to enlarge it |
It is because the spins of conduction electrons align them along the magnetic field. (Details see here and here). In short, in a magnetic, there is a spin precession and a spin-precession damping. Due to the spin-precession damping, the electron spin is aligned along the magnetic field.
Additionally to the spin alignment in a magnetic field, there is a spin relaxation or a spin re-alignment from one direction. The spin polarization of the electron gas of the conduction electrons is determined by a balanced of the spin pumping ( the spin alignment in one direction) and the spin relaxation (spin re-alignment from one direction). Details see here.
There are two contribution to the magnetization increase. The first main contribution is the increase of the total spin of the conduction electrons due to the increase of the number of the spin-polarized conduction electrons. Since the spin of each spin-polarized conduction electron is aligned along the magnetization, the magnetization increase is linearly proportional to the increase number of the spin-polarized electrons and the spin of a conduction electrons. The second contribution is the spin of localized d-electrons.
(step 1) Calulation of the spin polarization of the electron gas induced by the magnetic field
(step 2) Calculation of the additional moment, which is originated due to the change of the spin polarization.
The detailed description is here and here. The following is the description in short.
Spin polarization in a metal of different type
a ferromagnetic metal
In a ferromagnetic metal, spins of all localized d-electrons are in one direction. The conduction electrons are spin-polarized. All conduction electrons can be divided into two groups: spin-polarized and spin-unpolarized electrons. In the group of spin-polarized electrons, spins of all electrons in one direction. In the group of spin-unpolarized electrons, the spin directions are equally distributed in all directions.
Spins of the localized d-electrons are in one direction because of the exchange interaction with a few neighbor electrons.
The reason why the conduction electrons are spin-polarized is:
1) Scattering between localized and conduction electrons. Therefore, spins of some conduction electrons become in the same direction as spins of localized electrons.
2) The sp-d exchange interaction between the localized and conduction electrons.
a non-magnetic metals
In a non-magnetic metal the conduction electrons are not spin-polarized. The spin directions of the conduction electrons are equally distributed in all directions and the total spin is zero.
In a magnetic field the conduction electrons become spin-polarized (See here).
Also, the spin-polarized electrons can be injected or they can diffuse from a ferromagnetic metal to a non-magnetic metal
Non-magnetic metals include paramagnetic and diamagnetic metals.
a diamagnetic metal
Localized electrons do not have spin. Therefore, there is no sp-d exchange interaction between the conduction electrons and localized d-electrons and the sp-d scatterings is spin- independent. As a result,, the spin life for conduction electrons in a diamagnetic metal is longest.
a paramagnetic metal
Localized electrons do have spin. The spin directions of the localized electrons are equally distributed in all directions and the total spin is zero.
In a magnetic field the localized electrons tend to align their spins along magnetic field. However, thermo- fluctuation realign the spins. The resulting spin distribution is described by the the Brillouin function.
|
Localized d-electron:
-They have a size of one atomic orbital.
-Their spin direction is determined mainly by the exchange interaction with close neighbor localized d-electrons.
-scatterings between neighbor localized electrons are rare;
-scatterings between localized and conduction electrons are rare;
Spin polarization is a balance between spin pumping and spin relaxation |
| arrows shows the spin-direction and the volume of balls is proportional to the number of the spin polarized electrons. The big ball shows all conduction spin-polarized electrons. |
| Spin pump by localized d-electrons are shown as electrons moving horizontally. The spins of d-electrons are in one direction. The conduction electrons with the same spin direction constantly created due the d-electron scatterings and the exchange interaction between conduction and d-electrons. |
| Spin relaxation are shown as electrons falling dawn. The spin relaxation is the electron conversion from group of spin-polarized electrons to group of the spin-unpolarized electrons |
| click on image to enlarge it |
Conduction (delocalized) sp-electrons:
-They have a size of a thousand and more atomic orbitals;
-scatterings between conduction electrons are very frequent;
-spin of each conduction electron frequently rotates after each frequent scattering (See here);
- total spin of all conduction electrons is nearly constant. It my decrease slowly within the spin relaxation time.
Spin of conduction electrons |
||||||
|
||||||
| click on image to enlarge it |
(simplification 1) Ignorance of the conservation law of the local spin and the local conservation of the time inverse symmetry.
In order to simplify the calculations, both The Pauli and Stoner models ignores the scatterings and the transformation of the spin during the frequent scattering. They are based on the fact that the total spin of all conduction electrons are conserved. Therefore,
|
||
Fig.1 Pauli paramagnetism. Classical explanation by the model of spin-up/spin-down bands. (left) Conduction electrons when there is no magnetic field. There is an equal number of spin-up and spin-down electrons. (right) Magnetic field is applied, The spin-down electrons have a larger energy. Since in equilibrium the Fermi energy is equal for spin-up and spin down bands, the spin-up band will be filled by a greater number of electrons. |
This classical model explains the paramagnetic susceptibility of a non-ferromagnetic metal due to the conduction electrons (For more details, see Kittel, Charles - Introduction to Solid State Physics page 433).
In the absence of a magnetic field there is an equal number of electrons in the spin-down and spin-up bands. Therefore, the net magnetization due to the conduction electrons is zero.
In a magnetic field the spin-up and spin-down electrons have different energy. The energy difference is
![]()
In order to make the Fermi energy equal for both bands, after a magnetic field is applied, some electrons from the spin-down band are flipped into the spin-up band. Therefore, the spin-up band will be filled by a greater number of electrons than the spin-down band. Due to the difference in the number of spin-up and spin-down electrons, the net magnetization of conduction electrons becomes non-zero.
At zero temperature the magnetization can be calculated as
![]()
where ![]() are the total number of conduction electrons with spin-up and spin-down, respectively. D is the density of states near the Fermi energy.
are the total number of conduction electrons with spin-up and spin-down, respectively. D is the density of states near the Fermi energy.
|
||
Fig.2 The Pauli paramagnetism explained from the model of TIA/TIS assemblies. (left) Conduction electrons when there is no magnetic field. All electrons in TIS assembly (right) When magnetic field is applied some electrons are converted into TIA assembly. |
In the absence of a magnetic field all conduction electrons of a non-magnetic metal are in the TIS assembly. Even though there are “spin” states in the TIS assembly, the total magnetic moment of the TIS assembly is zero, because this assembly is time-inverse symmetrical. The magnetic moment is time-inverse asymmetrical and only time- inverse asymmetrical objects may have non-zero magnetic moment. Therefore, the total magnetic moment of an electron gas may be non-zero only in the case when some electrons are in the TIA assembly.
In a magnetic field there is a conversion of “spin” states from the TIS assembly into the TIA assembly at the rate (Eq.20 here)
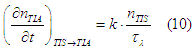
where ![]() is the precession damping time and k=4/3 in the case of a weak magnetic field and k is smaller than 4/3 in the case of a larger magnetic field (See condition (13) here).
is the precession damping time and k=4/3 in the case of a weak magnetic field and k is smaller than 4/3 in the case of a larger magnetic field (See condition (13) here).
There is a back conversion of “spin” states from the TIA assembly into the TIS assembly due to spin relaxation mechanisms. The rate of this conversion is proportional to the number of electrons in the TIA assembly

where ![]() is the spin life time.
is the spin life time.
In equilibrium the rate of conversion of electrons from the TIS assembly into the TIA assembly should be equal to the rate of reverse conversion

Pauli Paramagnetism |
|||||||||
|
|||||||||
| click on image to enlarge it |
From Eq. (12) the equilibrium number of electrons in the TIA assembly can be calculated as
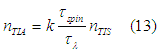
The magnetic moment per “spin” state of the TIA assembly is
![]()
where g is the g-factor, ![]() is the Bohr magneton.
is the Bohr magneton.
The induced magnetic moment of an electron gas is equal to the total magnetic moment of the TIA assembly and it can be calculated as
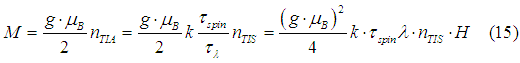
|
||
Fig.3. Animated pictures. Comparison of mechanisms responsible for the Pauli paramagnetism as represented by the model of the spin-up/spin-down bands (left) and the model of TIA/TIS assemblies (right) |
Therefore, the induced magnetic moment of an electron gas is calculated as
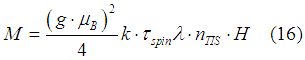
where lambda is a phenomenological damping parameter of spin precession. The number of “spin” states in the TIS assembly ![]() should be calculated using the spin statistics as described here.
should be calculated using the spin statistics as described here.
In the case of a weak magnetic field (See condition (13) here) the induced magnetization is linearly proportional to the magnetic field. This means that in this case the permeability does not depend on the intensity of the magnetic field. For example, in the case of a metal with constant density of states (See Eq. (18b) here), the induced magnetic moment of the electron gas is calculated as
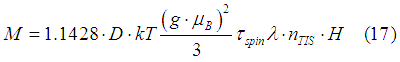
where D is the density of states.
For a stronger magnetic field the permeability decreases when the magnetic field increases. This is because the coefficient k decrease (See Fig.7 here) and the number of “spin” states in the TIS assembly decreases. The TIS assembly has a smaller number of "spin" states, because some “spin” states have been converted into the TIA assembly.
In the case of an even larger magnetic field, nearly all “spin” states may be converted into the TIA assembly (the metal is near 100 % spin polarized) and the induced magnetization saturates at a value
![]()
where ![]() is the total number of spin states.
is the total number of spin states.
See wikipedia explanation here
According to the Stoner model, in a ferromagnetic metal the spin direction of all conduction electrons is either along or opposite to the metal magnetization direction. An exchange interaction has split the energy of states with different spins, and states near the Fermi level are spin-polarized.
The density of spin-down states is smaller than the density of spin-up states. Because of this difference, the conductivity of a ferromagnetic metal becomes spin-dependent, which leads to several interesting effects for transport in the ferromagnetic metal such as a charge accumulation, the shortening of spin diffusion length and the blocking of spin diffusion (See details see here).
|
||
Fig.4. Conduction electrons in a ferromagnetic metal. (left) Model of spin-up/spin-down bands. The blue band is the majority band and the yellow band is the minority band. (right) Model of TIA/TIS assemblies. The blue assembly is the TIA assembly and the yellow assembly is the TIS assembly . |
The difference of the density of states for minority and majority spins in ferromagnetic metals has been verified experimentally.
The Stoner model is based on the classical model of spin-up/spin-down bands.
In a ferromagnetic metal the d-orbitals are partially filled and electrons can be divided into local d-electrons with non-zero spin and conduction sp-electrons. There is an exchange interaction between d-electrons, which aligns spins of all d-electrons in one direction.
Also, there is an exchange interaction between the local d-electrons and the conduction sp-electrons, which leads to the conversion of “spin” states from the TIS assembly into the TIA assembly. In addition, there is a back conversion from the TIA assembly into the TIS assembly, because of a finite spin life time. In equilibrium the conversion of electrons from the TIS to the TIA assembly is balanced by back conversion.
In equilibrium the conduction electrons in a ferromagnetic metal are in one TIA assembly and in one TIS assembly.
The conversion rate from the TIS assembly to the TIA assembly, which originates from the exchange interaction between sp- and d-electrons, is proportional to the number of “spin” states in the TIS assembly (See Eq.(21) here)
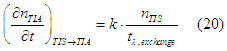
where ![]() is the precession damping time for exchange interaction and k=4/3 in the case of a weak exchange field and k is smaller than 4/3 in of case of a larger exchange field (See condition (13) here).
is the precession damping time for exchange interaction and k=4/3 in the case of a weak exchange field and k is smaller than 4/3 in of case of a larger exchange field (See condition (13) here).
The electrons are converted from the TIA assembly into the TIS assembly due to spin relaxation mechanisms. The rate of this conversion is proportional to the number of electrons in the TIA assembly
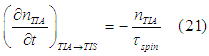
where ![]() is the spin life time.
is the spin life time.
In equilibrium the rate of conversion of electrons from the TIS assembly into the TIA assembly should be equal to the rate of the reverse conversion

From Eq. (12) the relative number of “spin” states in the TIA assembly to the number of “spin” states in the TIS assembly can be calculate as
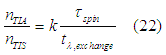
The spin polarization of conduction electrons in a ferromagnetic metal can be calculated as
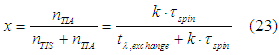
Spin precession |
||||||
|
||||||
| click on image to enlarge it |
Distribution of "spin" states in TIA and TIS assemblies can be calculated by the spin statistic.
 or here
or here 
I will try to answer your questions as soon as possible