Dr. Vadym Zayets
v.zayets(at)gmail.com
My Research and Inventions
click here to see all content |

Dr. Vadym Zayetsv.zayets(at)gmail.com |
|
 |
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionTransport Eqs.Spin Proximity/ Spin InjectionSpin DetectionBoltzmann Eqs.Band currentScattering currentMean-free pathCurrent near InterfaceOrdinary Hall effectAnomalous Hall effect, AMR effectSpin-Orbit interactionSpin Hall effectNon-local Spin DetectionLandau -Lifshitz equationExchange interactionsp-d exchange interactionCoercive fieldPerpendicular magnetic anisotropy (PMA)Voltage- controlled magnetism (VCMA effect)All-metal transistorSpin-orbit torque (SO torque)What is a hole?spin polarizationCharge accumulationMgO-based MTJMagneto-opticsSpin vs Orbital momentWhat is the Spin?model comparisonQuestions & AnswersEB nanotechnologyReticle 11
|
Groups of spin-polarized and spin-unpolarized electrons
Magnetism of electron gasAll conduction electrons in an electron gas can be divided into two groups of spin-polarized and spin-unpolarized electrons. The total spin of electrons of the group of spin-unpolarized electrons is zero. The spin directions of electrons of the group of spin-unpolarized electrons is equally distributed in all directions. All spins of electrons of the group of the spin-polarized electrons are in the same direction. The total spin of this group is non-zero.The properties of electrons in the groups of spin-polarized and spin-unpolarized electrons are different: (1) energy distributions are different (2) the transport properties are different. For example, the electrical conductivities for groups of spin-polarized and spin-unpolarized electrons are different. Because of this difference the electrical conductivity becomes spin-dependent (the magneto-resistance effect).
|
Experimental method of measurement of spin polarization is described here |
 |
| Click on image to enlarge it. |
Because many properties of spin-polarized and spin-unpolarized electrons are different. For example, such properties:
(1) Energy distribution
(2) Electron transport
(3) tunneling
(4) magnetic properties
![]() Conduction electrons occupy quantum states. Each quantum state can be occupied either by one electron or two electrons of opposite spins
Conduction electrons occupy quantum states. Each quantum state can be occupied either by one electron or two electrons of opposite spins
Quantum states of conduction electrons:
(1) Half-filled states. They are filled only by one electron. They are occupied by spin-polarized and spin-unpolarized electrons. Mainly electrons of these states contribute to the charge and spin transport.
(2) Full-filled states. They are filled by two electrons of opposite spins. They are occupied by spin-inactive or deep-level electrons.
(3) Empty state, which are not filled at all.
All conduction electrons can be divided into 3 groups:
 Group (2): Spin-unpolarized electrons
Group (2): Spin-unpolarized electrons Group (3): Spin-inactive electrons
Group (3): Spin-inactive electrons Group of spin-polarized electrons |
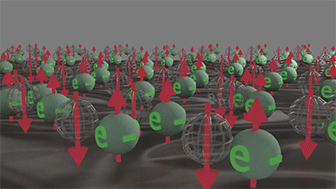 |
| Spins of all electrons are in one direction. All electrons occupy only half-filled states, in which one place is empty. Click on image to enlarge it. |
It consists of electrons, which occupy a half-filled states. Spins of all electrons are directed in the same direction.
The total spin of this group is always non-zero.
The time-inverse symmetry is broken for this group.
![]() Can two groups of spin-polarized electrons with different spin directions coexist at the same time and at the same place?
Can two groups of spin-polarized electrons with different spin directions coexist at the same time and at the same place?
In principle, yes. However, within a very short time the scattering convert two group with two directions of spin directions into one group with one spin direction (See here). It takes only a few scatterings and about a hundred femtoseconds for the conversion.
In a semiconductor the spin-polarized electrons and spin-polarized holes may have different spin directions for a long time. Because of small scattering probability between electron of conduction band, which have s- like symmetry, and electrons of the valence band, which have the p-like symmetry, the different spin directions of holes and electrons can coexists for a few microseconds.
Group of spin-unpolarized electrons |
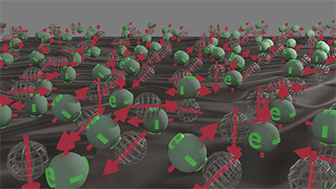 |
| Spin directions are equally distributed in all directions. All electrons occupy only half-filled states, in which one place is empty. Click on image to enlarge it. |
It consists of electrons, which occupy a half-filled states. Spins of these electrons are equally distributed in all directions.
The total spin of this group is zero.
The time-inverse symmetry is not broken for this group.
It consists of electrons, which occupy a full-filled states. The spin direction of these electrons is not defined.
The total spin of this group is zero.
The time-inverse symmetry is not broken for this group.
![]() The spin-polarized electrons, spin-unpolarized electron and spin-inactive electrons have different energy distributions.
The spin-polarized electrons, spin-unpolarized electron and spin-inactive electrons have different energy distributions.
For more details See Spin Statistics
For example, in case when a state is already occupied by one electron with spin-up, it is only possible for another electron to be scattered into this state only if it has spin-down. The spin of the scattered electron should be exactly opposite to the spin of the electron, which is already occupying the state. In the group of the spin-polarized electrons, all electrons have the spins in one direction. Therefore, there are no any scatterings between states occupied by spin-polarized electrons. In the group of spin-polarized electrons, the spins are distributed equally in all directions. Therefore, there is a probability between 0 and 1 that a spin-unpolarized electron is scattered into a state occupied either by spin-polarized electron or by another spin-unpolarized electron. A full-filled state, which is occupied by two electrons, has spin zero and no defined spin direction. Therefore, electrons of this state have no spin limitations for a scattering. The different scattering probabilities determine the numbers of spin-polarized and spin-unpolarized electrons and electrons in full-filled states
The spin polarization sp of the electron gas is defined as a ratio of the number of spin-polarized electrons to the total number of the spin-polarized and spin-unpolarized electrons:
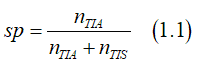
where nTIA and nTIS are the numbers of spin-polarized and spin-unpolarized electrons, respectively.
The spin polarization is determined by a balance between the spin pumping and the spin relaxation. The spin pumping is the conversion of electrons from groups of spin-unpolarized electrons into the group of the spin-polarized electrons. The spin relaxation is the conversion in the opposite direction.
A. Both mechanisms the spin pumping and spin relaxation are related to a spin rotation. The spin pumping is the alignment of spins along one direction. The spin relaxation is re-alignment of spins from one direction. The spin of "full" state is zero and the spin-inactive electrons, which occupy the "full" states", do not have any defined spin direction. Therefore, their spin cannot be rotated and they cannot contribute either to the spin pumping or to the spin relaxation.
Spin polarization is a balance between spin pumping and spin relaxation |
||||||
|
||||||
| arrows shows the spin-direction and the volume of balls is proportional to the number of the spin polarized electrons. The big ball shows all conduction spin-polarized electrons. | ||||||
Spin pump source is shown as antenna. Spin pump creates electrons with spin directed in one direction. A faster spin pumping makes the spin polarization larger. The source of the spin pumping is an external magnetic field, interaction with localized d-electron and the absorption of a circular-polarized light. The figures show the case of the spin pumping by localized d-electrons. The conduction electrons with the same spin direction constantly created due the d-electron scatterings and the exchange interaction between conduction and d-electrons. |
||||||
| Spin relaxation are shown as electrons falling dawn. The spin relaxation is the electron conversion from group of spin-polarized electrons (spin aligned in one direction) to group of the spin-unpolarized electrons (spin is not aligned) | ||||||
| click on image to enlarge it |
![]() Spin-pumping rate:
Spin-pumping rate:
The spin pumping describes the conversion of electrons from the group of the spin-polarized electrons into the group of spin-unpolarized electrons. The conversion rate of the spin-pumping is described as
where tpump is the spin pumping time.
mechanism (1): the sp-d exchange interaction between conduction electrons and localized d-electrons: (details see here)
mechanism (2): sp-d scatterings;(details see here)
The spins of the d-electrons are aligned in one direction. When a d-electron are scattered and becomes a conduction electron, more spins of conduction become align along the magnetization (along d-electrons)
mechanism (3): precession damping in a magnetic field; (details see here)
There is a precession of spins of conduction electrons in a magnetic field. Additionally, the spins are aligns along the magnetic field. (details see here)
mechanism (4): spin-dependent scatterings & spin accumulation due to Spin Hall effect; (details see here)
When an electrical current flows in a ferromagnetic metal, the amount of conduction electrons to the left and to the right direction with respect to the current direction may be different. The difference depends on spin direction of the conduction electrons. As a result, spin-polarized electrons are accumulated at sides of the ferromagnetic wire
mechanism (5): spin injection and spin diffusion; (details see here and here)
The spins diffuses from a region of a higher spin polarization of the electron gas to a region of a smaller spin polarization. This effect is called the spin proximity effect. Additionally, the spins are drifted in electrical current from a region of a higher spin polarization of the electron gas to a region of a smaller spin polarization. This effect is called the spin injection.
mechanism (6): optical spin pumping ; (details see here)
When a metal is illuminated by circular- polarized light, the spin of photon is transferred to the spin of the electron gas and the spin polarization of the electron gas may increase.(details see here)
mechanism (7): Ordinary Hall and Anomalous Hall effects; (details see here and here)
In magnetic field the electrical current is turned slightly from a strait direction along an electrical field due to the Hall effect. The electrons and holes are accumulated at opposite sides of wire due to the opposite Lorentz force (the ordinary Hall effect) or due to directional dependence of scatterings (Anomalous Hall effect). In a ferromagnetic metal the amount of spin polarized holes and electrons may be slightly different. As a result, the spin polarization slightly increase at one side of wire and decreases at another side.
![]() Spin-relaxation rate:
Spin-relaxation rate:
The spin damping describes the conversion of electrons from the group of the spin-polarized electrons into the group of spin-unpolarized electrons. The conversion rate of spin-relaxation can be described as
where trelax is the spin relaxation time.
![]() Mechanisms of spin-relaxation:
Mechanisms of spin-relaxation:
mechanism (1): spin-dependent scatterings
After a spin-dependent scattering the spin direction of a spin-polarized conduction electron can be rotated from its initial direction
mechanism (2): incoherent spin precession in a spatially inhomogeneous magnetic field:
The direction of the internal magnetic field in a ferromagnetic metal may varies slightly from a place to place. Such inhomogeneities causes incoherent precession and the spin relaxation. (detail see here)
mechanism (3): local fluctuation of magnetization direction:
case 1:A ferromagnetic metal (spins of localized d-electrons are parallel). Locally a spin of the d-electron may slightly rotate out from one direction due to a thermal fluctuation. A small spin rotation reduces the strength of the spin pumping (Eq. (1.5)). A larger rotation causes the spin relaxation (Details see here)
case 2:A paramagnetic metal (spins of localized electrons are equally distributed in all direction). The sp-d exchange and the sp-d scatterings create spin-polarized conduction electrons. However, their spin directions are different from one local place to another place. This causes a substantial spin relaxation due to the spin torque (See here)
mechanism (4): Spin diffusion through a boundary between two materials:
The spin polarization and spin direction may be different in two contacting metals. As a result, the spin diffusion current and the spin-torque current flow through the interface. Both currents induces the relaxation.
mechanism (5): Spin diffusion through a domain wall:
The magnetization direction are different from a domain to a domain. The spin direction of spin-polarized electrons are different from a domain to a domain. A the spin-torque current flows between regions of different directions of the spin polarization. The spin-torque current induces a substantial spin relaxation. (The spin-torque current occurs due to the electron diffusion. More details see here)
Spin polarized conduction electrons in a ferromagnetic metal |
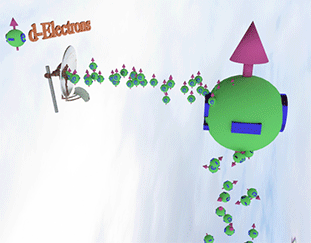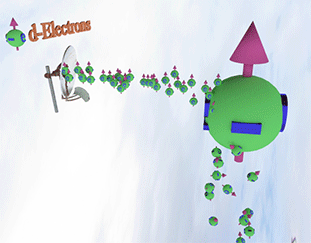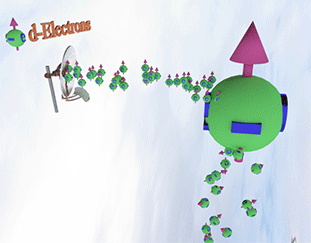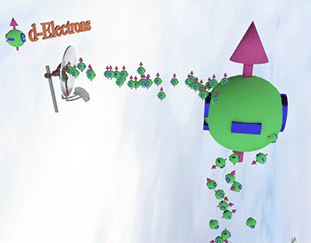 |
| arrows shows the spin-direction and the volume of balls is proportional to the number of the spin polarized electrons. The big ball shows all conduction spin-polarized electrons. |
Spin pump source is shown as antenna. Spin pump creates electrons with spin directed in one direction. A faster spin pumping makes the spin polarization larger. The source of the spin pumping in a ferromagnetic metal is the localized d-electrons. source is shown as antenna. Spin pump creates electrons with spin directed in one direction. A faster spin pumping makes the spin polarization larger. The source of the spin pumping is an external magnetic field, interaction with localized d-electron and the absorption of a circular-polarized light. The conduction electrons with the same spin direction constantly created due the d-electron scatterings and the exchange interaction between conduction and d-electrons. |
| Spin relaxation are shown as electrons falling dawn. The spin relaxation is the electron conversion from group of spin-polarized electrons (spin aligned in one direction) to group of the spin-unpolarized electrons (spin is not aligned). A larger the spin relaxation makes the spin polarization smaller. |
| click on image to enlarge it |
In equilibrium there is a balance between the spin pumping and the spin relaxation, which is described by the condition:
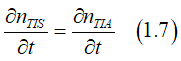
Substitution Eqs.(1.5) and (1.6) into Eq. (1.7) gives

Substituting Eq.(1.8) into Eq.(1.1) gives the equilibrium spin polarization sp0 as
Mean-free path or effective length of spin-polarized and spin-unpolarized electrons |
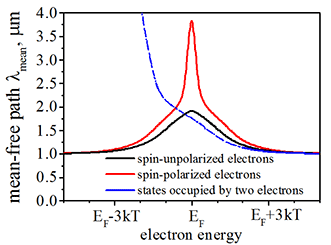 |
The mean-free path or effective length of spin-polarize, spin-unpolarized electrons and electrons of full-filled states. Spin polarization is 85%. Average distance between defects is 1 µm. The mean-free path is calculated here. Click on image to enlarge it. |
![]() The spin-polarized electrons, spin-unpolarized electron and electrons, which occupy full-filled states, have very different mean-path (effective length) . The mean-free path depends on electron energy. More details is here
The spin-polarized electrons, spin-unpolarized electron and electrons, which occupy full-filled states, have very different mean-path (effective length) . The mean-free path depends on electron energy. More details is here
A. It is because of a different scattering probability for different state. In order to be scattered from a state the electron free space where to be scattered. For example, at energy sufficiently below the Fermi energy near all states are filled by two electrons of opposite spins (Fig.35). Therefore, near there is no any unoccupied state where an electron could scattered into. Therefore, in this case the life time of full-filled state is very long and the mean-free path is long as well. In contrast, an electron from a half-filled state can be scattered into empty state and a higher energy and an electron from full-filled state can be scattered into the half-filled state. Therefore, it is always many states are available for a scattering from/into a half-filled state. It makes the life time of half-filled state is short and the mean-free path is short as well.
Because of the wave nature of an electron, the effective length of an electron equals to the mean-free path. (See here)
![]() Due to difference of the effective lengths of a half-filled state and a full-filled state, in a semiconductor a "hole" has an effective plus charge and an "electron" has an effective minus charge. Even though both the "holes" and "electrons" are conduction electrons in the semiconductor. (See here)
Due to difference of the effective lengths of a half-filled state and a full-filled state, in a semiconductor a "hole" has an effective plus charge and an "electron" has an effective minus charge. Even though both the "holes" and "electrons" are conduction electrons in the semiconductor. (See here)
It is because of frequency of scatterings. The scatterings between localized electrons are rare. The scatterings between delocalized (conduction) electrons are frequent.
Localized electrons
The localized electrons are the orbital electrons of atom. They have size about the size of an atom. They stay for a long time at one atom and they do not move along metal.
The wave function of localized electrons are not overlapped. Only wave functions of a few neighbor localized are overlapped. This reason why the scatterings between localized electrons are random.
The spin directions of localized electrons are determined by the exchange interaction with neighbor localized electrons. Often spins of neighbor localized electrons are aligned parallel (ferrimagnetically) or antiparallel (antiferromagnetic). Also, they can be aligned at other angles (for example, in a domain wall)
![]() Energy distribution of localized electrons: sharp narrow peaks in the density of states (DOS)
Energy distribution of localized electrons: sharp narrow peaks in the density of states (DOS)
![]() Distribution of spin directions of localized electrons: A defined spin direction at each atomic site.
Distribution of spin directions of localized electrons: A defined spin direction at each atomic site.
![]() Optical and magneto-optical response of localized electrons: Sharp resonance-like peaks
Optical and magneto-optical response of localized electrons: Sharp resonance-like peaks
![]() Localized electrons can support a spin wave ( a magnon)
Localized electrons can support a spin wave ( a magnon)
Delocalized ( conduction) electrons
The delocalized electrons spreads over distance of thousands or even millions atoms. Their size is not fixed, but determined by number of defects and temperature of a metal.
The wave function of conduction electrons are strongly overlapped. At one point of space it could be millions of conduction electrons at the same time. This reason why the scatterings between delocalized electrons are very frequent.
The spin directions of localized electrons are determined by the scatterings between them. The electrons can be spin-polarized or spin-unpolarized (see above). It could be only spin-direction of spin-polarized electrons (there is only one group of spin-polarized electrons). In the case if spin direction of spin-polarized electrons is different at different places of a sample, the spin-torque current flows between these two places. The spin-torque current is trying to realign spin direction at different places in one direction.
There are two types of conduction electrons: running-wave electrons and standing-wave electrons (see here). Mainly all conduction electrons are running-wave electrons. They constantly move along crystal. In vicinity of a defect or an interface, some conduction electrons become standing-wave electrons. The standing-wave electrons stay at one place and do not move along crystal. The increasing of the number of the standing-wave electrons significantly changes properties of the spin transport in a metal (See here)
![]() Energy distribution of delocalized electrons: a broad smooth distribution without any peaks or sharp features (band-type DOS distribution)
Energy distribution of delocalized electrons: a broad smooth distribution without any peaks or sharp features (band-type DOS distribution)
![]() Distribution of spin directions of delocalized electrons: There is no any local spin. There is a spin distribution for whole electron gas. There are 3 groups of electrons: (1) group 1 is spin-polarized electrons; (2) group 2 is spin-unpolarized electrons; (3) group 3 is spin-inactive deep-level electrons;
Distribution of spin directions of delocalized electrons: There is no any local spin. There is a spin distribution for whole electron gas. There are 3 groups of electrons: (1) group 1 is spin-polarized electrons; (2) group 2 is spin-unpolarized electrons; (3) group 3 is spin-inactive deep-level electrons;
![]() Optical and magneto-optical response of delocalized electrons: A broad featureless spectrum
Optical and magneto-optical response of delocalized electrons: A broad featureless spectrum
![]() Delocalized electrons cannot support a spin wave ( a magnon)
Delocalized electrons cannot support a spin wave ( a magnon)
![]() The d-electrons in a ferromagnetic metal are localized with some weak band-type features.
The d-electrons in a ferromagnetic metal are localized with some weak band-type features.
A. Not much. The d-electrons have some component of sp- spacial symmetry, but still the d-electrons remain fully localized. (Nearly similar as it would not be any hybridization). The conduction electrons have some component of d- spacial symmetry, but the conduction electrons remain fully delocalized. Any electron is either localized (electron length is short) or delocalized (electron length is long). An electron cannot have simultaneously one short part and one long part.
|
 Ferromagnetic metals
Ferromagnetic metalsLocalized electrons
All spins of localized electrons are in one direction
Conduction electrons
All conduction electrons are in three groups: group of spin polarized, group of spin-unpolarized electrons and group of spin-inactive electrons.
Mutual spin directions of conduction and localized electrons
d-d exchange interaction and conduction electrons
importance of standing-wave conduction electrons for the d-d exchange interaction
 Antiferromagnetic metals (consist of one element)
Antiferromagnetic metals (consist of one element)Localized electrons
There are equal amounts of spins, which are directed antiparallel. The total magnetization of an antiferromagnetic metal is zero.
Conduction electrons
All conduction electron are spin-unpolarized. The spin polarization of electron gas is zero.
d-d exchange interaction and conduction electrons
 Compensated ferromagnetic metal or Antiferromagnetic metals (consist of two or more elements)
Compensated ferromagnetic metal or Antiferromagnetic metals (consist of two or more elements)Localized electrons
There are equal amounts of spins, which are directed antiparallel. The total magnetization is zero. Usual
Conduction electrons
All conduction electrons are in three groups: group of spin polarized, group of spin-unpolarized electrons and group of spin-inactive electrons.
Example: compensated ferromagnet FeTbB
 Non-magnetic metals
Non-magnetic metalsLocalized electrons
in a diamagnetic metal the localized electrons do not have spin. In a paramagnetic metal, the spins of localized electrons are equally distributed in all directions.
Conduction electrons
All conduction electron are spin-unpolarized. The spin polarization of electron gas is zero.
spin diffusion and spin injection
Magnetization of localized d-electrons in metals |
||||||||||||
|
||||||||||||
Click on image to enlarge it. |
A. Absolutely not. The separation of all conduction electrons into the conduction electrons into groups of the holes and the electrons is possible only because the exchange of electrons (scatterings) between two groups is very seldom. The scatterings are seldom because the holes and the electrons in a semiconductor belong to bands of different symmetry (s-symmetry for conduction band and p-symmetry for the valence band). In contrast, the exchange of electrons (scatterings) between groups spin-polarized and spin-unpolarized electrons is very frequent. The frequent scatterings keep numbers of electron in each group to be constant. It is why it is possible to separate all conduction electrons into spin-polarized and spin-unpolarized groups. The reason is very different (even opposite) from that used to separate the holes and the electrons.
Because of the rare exchange of electrons between the conduction and valence bands, the hole and the electrons may have different Fermi energies and the chemical potentials. Because of this property it is possible to fabricate a semiconductor laser and a bipolar transistor (see here) . In contrast, the spin-polarized and spin-unpolarized electrons always have the same Fermi energy.
![]() Note: The model of spin-up/spin-down bands assumes that there is no exchange of electrons between groups of the spin-polarized and unpolarized electrons. Additionally, it assumes that the spin-polarized and unpolarized electrons may have different Fermi energies. In this model, the different Fermi energies, different chemical potentials and different conductivities are used for electrons of spin-down and spin-up bands (See here). It is a rather rough assumption. This assumption can be used in some cases, but it has its limits. The validity of this assumption for each specific case should be carefully verified.
Note: The model of spin-up/spin-down bands assumes that there is no exchange of electrons between groups of the spin-polarized and unpolarized electrons. Additionally, it assumes that the spin-polarized and unpolarized electrons may have different Fermi energies. In this model, the different Fermi energies, different chemical potentials and different conductivities are used for electrons of spin-down and spin-up bands (See here). It is a rather rough assumption. This assumption can be used in some cases, but it has its limits. The validity of this assumption for each specific case should be carefully verified.
![]() The electron gas is the gas of the conduction electrons. It is the gas, because the conduction electrons strongly interact with each other. The conduction electrons are scattered between their quantum states very frequently. A conduction electron stays at one quantum state only for about 10-100 femtoseconds ( 10-14s - 10-13 s). Then, it is scattered to another state. The wave function of each conduction electron overlaps with wave functions of billions of other conduction electrons. Therefore, all properties of conduction electrons are very collective and the the gas of the conduction electrons should be considered as an individual subject with its own properties. The properties of individual conduction electrons should be described by a distribution. (e.g. the energy distribution or the distribution of the spin directions).
The electron gas is the gas of the conduction electrons. It is the gas, because the conduction electrons strongly interact with each other. The conduction electrons are scattered between their quantum states very frequently. A conduction electron stays at one quantum state only for about 10-100 femtoseconds ( 10-14s - 10-13 s). Then, it is scattered to another state. The wave function of each conduction electron overlaps with wave functions of billions of other conduction electrons. Therefore, all properties of conduction electrons are very collective and the the gas of the conduction electrons should be considered as an individual subject with its own properties. The properties of individual conduction electrons should be described by a distribution. (e.g. the energy distribution or the distribution of the spin directions).
In contrast, the localized d-electrons are very individual and they do not make a gas. The wave function of each d-electron overlaps only with the wave functions of a few neighbors d-electrons. As a result, a d-electron may possess some individual property like the spin direction. For example, in an antiferromagnetic metal, one d-electron may have the spin-up direction, but its neighbor d-electron may have the spin-down direction. They both have a specific spin direction and the spin direction retains in a long time. The case of an electron in the electron gas is very different. Only a distribution may be constant in time. Even though the number of spin-polarized and spin-unpolarized electrons may not change in time, but one individual conduction electron (an electron, which occupies some specific quantum state) may belong to the group of the spin-polarized electrons, but next femtosecond it may be scattered into the group of the spin-unpolarized electrons. Meanwhile, somewhere some spin-unpolarized electron is scattered in the opposite direction. The individual properties of a conduction electrons are smeared over the their collective properties. Therefore, the electron gas is defined and used.
I am sorry if there is some confusion in my explanation. Two groups of spin polarized electrons cannot coexist together at same time at the same place. The scatterings mix up these two group into one group very quickly. However, the group of the spin-polarized electrons and group of the spin- unpolarized electrons do coexist together. Even though the electrons are scattered between these two groups, the numbers of electrons in each group remains unchanged due to the spin conservation law.
The conduction electrons are similar in a ferromagnetic and paramagnetic metals. In both metals the conduction electrons can be either spin- polarized or spin- unpolarized. In any metal there is a force or effect, which disaligns the spins of the conduction electron from be aligned in one direction (this spin- disalignment force is called the spin relaxation). Therefore, the conduction electrons can be spin- polarized only if there is a force, which aligns the electron spins along one direction (this spin- alignment force is called the spin pumping). In a spin- polarized electron gas, the spin pumping and spin relaxation balance each other.
In a paramagnetic metal there is no spin pumping, but there is the spin relaxation. As a result, in a paramagnetic metal, the electron gas is not spin- polarized.
In a ferromagnetic metal, there is a spin pumping. The spins of conduction electrons are aligned along the spins of localized d- electrons due to the exchange interaction, scattering between conduction and localized electrons and an intrinsic magnetic field, which exists along aligned spins of localized d-electrons. Due to the existing of the spin pumping, the conduction electrons in a ferromagnetic metal are spin-polarized.
I would like emphasize once more, the conduction electrons and their spin properties in a ferromagnetic and paramagnetic metals are similar. All conduction electrons are divided into 3 groups: (group 1. Spin- polarized electrons). Electron fills only one of two places of a quantum states. All spins are directed in one direction. (group 2. Spin polarized electrons). Electron fills only one of two places of a quantum states. Spins are equally distributed in all directions. (group 3. Spin inactive electrons). Two electrons of opposite spins fill two places of a quantum states.
A. The spin (the absolute value =1/2 and the spin direction) is absolutely defined for each conduction electrons independently whether the electron belongs to the group of spin-polarized or spin- unpolarized electrons.
 The conservation laws of the spin, energy and charge are the strongest conservation laws in our Universe. For example, the Black Hole separates itself from the outside world and unreachably locks all information inside itself except its Mass, Charge and Spin
The conservation laws of the spin, energy and charge are the strongest conservation laws in our Universe. For example, the Black Hole separates itself from the outside world and unreachably locks all information inside itself except its Mass, Charge and Spin
Similarly as each electron can be distinguished by its energy, each electron can be distinguished by its spin. Similarly as there is an energy distribution, there is a distribution of the spin directions (See here)
 There is always some symmetry, which corresponds to any specific conserved quantity. The spin describes the conservation of the time- inverse symmetry. This symmetry is an unremovable feature of any object in our Universe. Definitely, each conduction electron has a defined spin. Similarly as it has a defined energy and momentum.
There is always some symmetry, which corresponds to any specific conserved quantity. The spin describes the conservation of the time- inverse symmetry. This symmetry is an unremovable feature of any object in our Universe. Definitely, each conduction electron has a defined spin. Similarly as it has a defined energy and momentum.
I will try to answer your questions as soon as possible