Dr. Vadym Zayets
v.zayets(at)gmail.com
My Research and Inventions
click here to see all content |

Dr. Vadym Zayetsv.zayets(at)gmail.com |
|
 |
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers
more Chapters on this topic:IntroductionTransport Eqs.Spin Proximity/ Spin InjectionSpin DetectionBoltzmann Eqs.Band currentScattering currentMean-free pathCurrent near InterfaceOrdinary Hall effectAnomalous Hall effect, AMR effectSpin-Orbit interactionSpin Hall effectNon-local Spin DetectionLandau -Lifshitz equationExchange interactionsp-d exchange interactionCoercive fieldPerpendicular magnetic anisotropy (PMA)Voltage- controlled magnetism (VCMA effect)All-metal transistorSpin-orbit torque (SO torque)What is a hole?spin polarizationCharge accumulationMgO-based MTJMagneto-opticsSpin vs Orbital momentWhat is the Spin?model comparisonQuestions & AnswersEB nanotechnologyReticle 11
more Chapters on this topic:IntroductionScatteringsSpin-polarized/ unpolarized electronsSpin statisticselectron gas in Magnetic FieldFerromagnetic metalsSpin TorqueSpin-Torque CurrentSpin-Transfer TorqueQuantum Nature of SpinQuestions & Answers |
Spin Torque
Magnetism of electron gasThe spin torque occurs when the electron gas is already spin-polarized and a small amount of spin-polarized electrons of different spin direction are injected. As result, the spin direction of all spin-polarized electrons rotates toward the spin direction of the injected electrons.The spin torque is always accompanied by an additional spin relaxation.
|
| Spin Torque |
|---|
| injection of a tiny amount of spin-polarized electrons of different spin direction rotates the spin direction of a larger amount of existed spin polarized electrons towards spin direction of injected electrons |
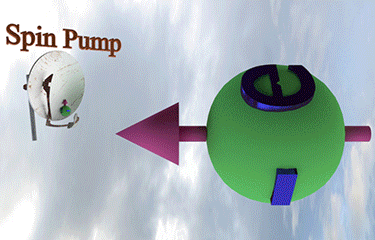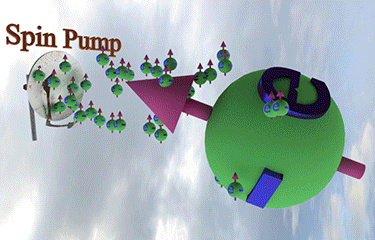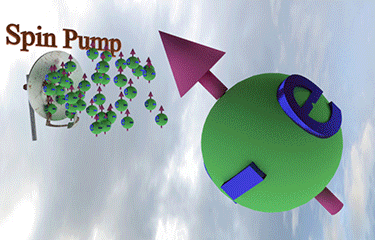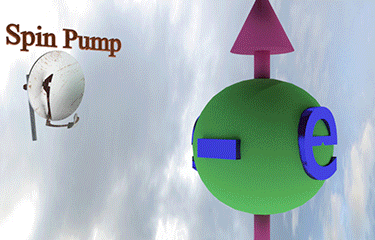 |
| arrows shows the spin-direction and the volume of balls is proportional to the number of the spin polarized electrons |
| click on image to enlarge it |
The spin torque causes an additional spin relaxation or conversion from group of spin-polarized electrons into group of spin-unpolarized electrons at the rate
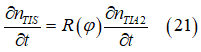
- Spin rotation of the spin-polarized electrons is directed towards the spin direction of the injected electrons
- The spin torque is largest in the case when the angle between the spin directions of existing and injected spin-polarized electrons is 67 degrees
- There is no spin torque in the cases when the spin directions of existing and injected spin-polarized electrons are parallel or anti parallel in respect to each other.
- The spin torque is linearly proportional to the injection rate of spin-polarized electrons
- Spin torque is always accompanied by spin relaxation (a conversion from the group of spin-polarized electrons into the group of spin-unpolarized electrons). The conversion rate is greater for a larger angle between the spin directions of existing and injected spin-polarized electrons.
- Injection of spin-polarized electrons increases the total number of spin-polarized electrons in the electron gas only when the angle between the spin directions of existing and injected spin-polarized electrons is smaller than 67 degrees. When the angle is larger than 67 degrees, the number of spin-polarized electrons decreases, because the spin relaxation, which is induced by the spin torque, becomes larger than the supplying rate of new spin-polarized electrons.


 Two or more Spin-Pumps
Two or more Spin-Pumps


![]() spin-pumping source (1): magnetic field (see here). Spin Direction: along magnetic filed
spin-pumping source (1): magnetic field (see here). Spin Direction: along magnetic filed
![]() spin-pumping source (2): sp-d exchange interaction and sp-d scattering (see here and here); Spin Direction: along magnetization
spin-pumping source (2): sp-d exchange interaction and sp-d scattering (see here and here); Spin Direction: along magnetization
 spin-pumping source (1): spin of a photon (see here).Spin Direction: along light propagation direction
spin-pumping source (1): spin of a photon (see here).Spin Direction: along light propagation direction
![]() spin-pumping source (2): sp-d exchange interaction and sp-d scattering (see here and here); Spin Direction: along magnetization
spin-pumping source (2): sp-d exchange interaction and sp-d scattering (see here and here); Spin Direction: along magnetization
| Spin Torque. Two spin pumps |
|---|
| When only one spin pump is on, the spin direction of electron gas (big ball) towards the spin pump direction. When both spin pumps are switched on, the the spin direction of electron gas is between spin directions of the spin pumps. |
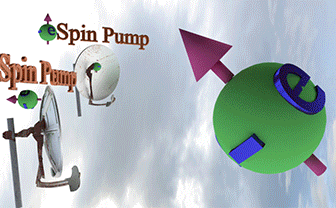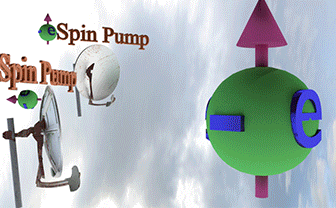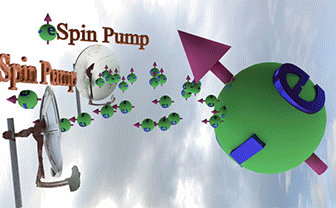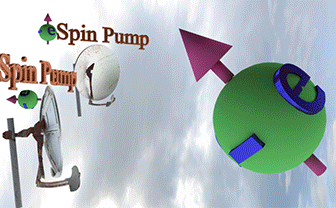 |
| Big ball shows a large number of spin-polarized electrons of electron gas. The small balls shows direction of injected spin-polarized electrons from two spin pumps. |
| Spin direction of the front spin pump is toward left. Spin direction of the backside spin pump is toward up. |
| arrows shows the spin-direction and the volume of balls is proportional to the number of the spin polarized electrons |
| click on image to enlarge it |
 spin-pumping source (1): spin Hall effect (see here) due to scatterings of spin-unpolarized electrons (see here). Spin Direction: along current
spin-pumping source (1): spin Hall effect (see here) due to scatterings of spin-unpolarized electrons (see here). Spin Direction: along current
![]() spin-pumping source (2): spin Hall effect (see here) due to scatterings of spin-polarized electrons (see here). Spin Direction: perpendicularly to the magnetization
spin-pumping source (2): spin Hall effect (see here) due to scatterings of spin-polarized electrons (see here). Spin Direction: perpendicularly to the magnetization
![]() spin-pumping source (3): sp-d exchange interaction and sp-d scattering (see here and here); Spin Direction: along magnetization
spin-pumping source (3): sp-d exchange interaction and sp-d scattering (see here and here); Spin Direction: along magnetization
 spin-pumping source (1): spin diffusion from a neighbor region
spin-pumping source (1): spin diffusion from a neighbor region
![]() spin-pumping source (2): sp-d exchange interaction and sp-d scattering (see here and here);
spin-pumping source (2): sp-d exchange interaction and sp-d scattering (see here and here);
![]() spin-pumping source (1): sp-d exchange interaction (scatterings) with spin-up localized electrons Direction: along magnetization
spin-pumping source (1): sp-d exchange interaction (scatterings) with spin-up localized electrons Direction: along magnetization
![]() spin-pumping source (2): sp-d exchange interaction (scatterings) with spin-down localized electrons; Direction: opposite to magnetization
spin-pumping source (2): sp-d exchange interaction (scatterings) with spin-down localized electrons; Direction: opposite to magnetization
| Effect of Spin-orbit torque (SOT) |
|---|
| There are two spin pumps. (spin pump 1): Localized d-electrons, which constantly creates spin-up conduction electrons. (spin pump 2): Due to Spin Hall effect, spin-left electrons is created. |
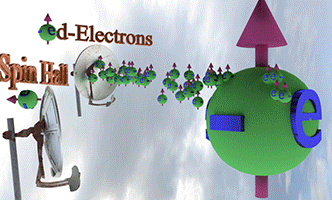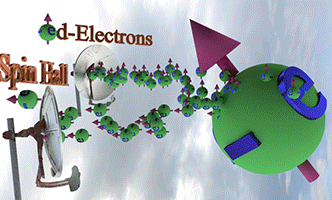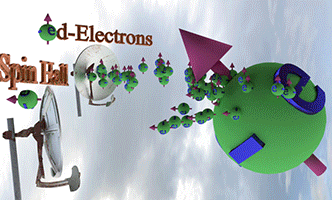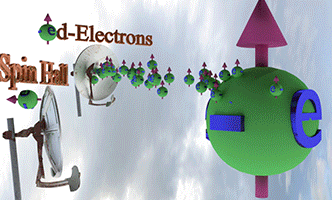 |
| Big ball shows a large number of spin-polarized electrons of electron gas. The small balls shows direction of injected spin-polarized electrons from two spin pumps. |
| Spin direction of the front spin pump is toward left. Spin direction of the backside spin pump is toward up. |
| arrows shows the spin-direction and the volume of balls is proportional to the number of the spin polarized electrons |
| click on image to enlarge it |
 The spin torque occurs as a result of the interaction of two groups of spin-polarized electrons.
The spin torque occurs as a result of the interaction of two groups of spin-polarized electrons.
The features of this interaction define the properties of the spin torque.
It is possible that at some moment in time in a metal there are two or more groups of spin-polarized electrons. However, within a very short time all spin-polarized electrons of two groups combine into one group of spin-polarized electrons and some electrons are converted into the group of the spin-unpolarized electron (additional spin relaxation).
![]() How long does it take for two groups of spin-polarized electrons with different spin directions to combine into one group with the same spin direction?
How long does it take for two groups of spin-polarized electrons with different spin directions to combine into one group with the same spin direction?
A. Between very quickly and almost immediately.
The time of interaction of two groups of spin-polarized electrons significantly depends on the relative number of electrons in each group. (almost immediately): In the case of the interaction of two groups with the same number of spin-polarized electrons, only (!!!) one scattering event 5 between each electron of two groups is sufficient to combine spin-polarized electron into one group with the same direction of spin. (very quickly): In the case of the interaction of two groups with a different number of spin-polarized electrons, one spin-polarized electron should experience several scattering event 5 until the two groups fully combine into one group with the same direction of spin. (See calculation below)
 Magnetic field is applied at some angle with respect to the spin direction of spin accumulated electrons.
Magnetic field is applied at some angle with respect to the spin direction of spin accumulated electrons.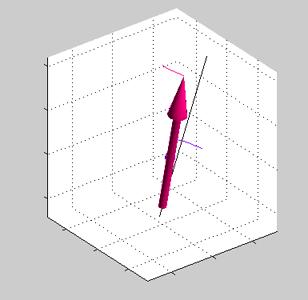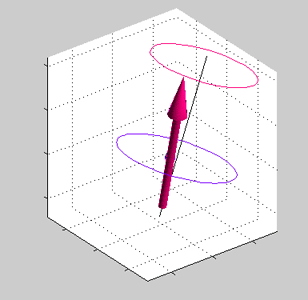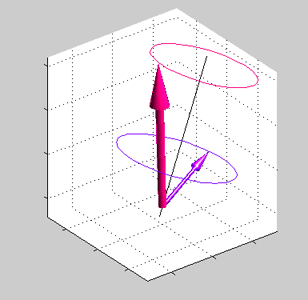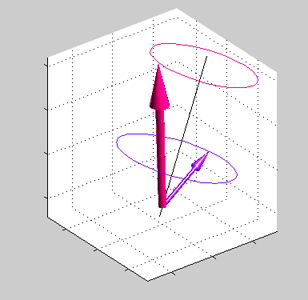 |
Precession of spins of the local d-electrons (red arrow) and spin-polarized conduction electrons (blue arrows) |
| Note: in an equilibrium there is no any precession of magnetic moment (See text) |
The spin torque affects the spin-polarized conduction electrons both in equilibrium and during the precession damping.
![]() spin torque in equilibrium: (a) magnitude of the spin polarization slightly changes (b) spin direction of spin-polarized electron slightly changes
spin torque in equilibrium: (a) magnitude of the spin polarization slightly changes (b) spin direction of spin-polarized electron slightly changes
![]() spin torque during precession damping: Pressing damping of spin-polarized conduction electrons increases during the precession damping
spin torque during precession damping: Pressing damping of spin-polarized conduction electrons increases during the precession damping
It is important for measurement of anisotropy field and the strength of perpendicular magnetic anisotropy (PMA) (See here)
A. Not always. When an external magnetic field is applied, the magnetization aligns itself very quickly (within 10-100 ns) along an effective magnetic field due to the precession damping. However, the direction of the effective magnetic field may be different from the direction of external and internal magnetic fields due to influence of the spin-orbit interaction and the exchange interaction:
(influence 1) spin-orbit interaction (SO)
(influence 2) exchange interaction
A magnetic field converts the electrons from the group of spin-unpolarized to the group of spin-polarized electrons. This effect is called the spin-pumping (See here and here). The spin direction of the converted electrons is along the magnetic field. In the case when the spin direction of the existed spin-polarized electrons in electron gas is different than the spin direction of the converted electrons, the existed spin-polarized electrons experience a spin torque, which rotates their spin towards the direction of the magnetic field.
Example: a magnetic field is applied in-plane to a ferromagnetic metal with a perpendicular magnetic anisotropy (PMA). This configuration is used to measure the anisotropy field (See here)
In the this case, in-polarization linearly depends on the magnetic field. The linear dependence makes easier to measure the strength of the PMA with a high precision (See here). The influence of the spin torque on this measurement should be taking into calculations.
A magnetic field constantly creates the spin-polarized electrons (spin pumping). The spin direction of these created spin-polarized electrons is along the magnetic field. When direction of the magnetic field is along the spin direction of existed spin-polarized electrons, the spin polarization of the electron gas in the magnetic field increases (experiment is here). When direction of the magnetic field is opposite to the spin direction of existed spin-polarized electrons, the spin polarization of the electron gas in the magnetic field decreases. For other angles of the magnetic field, the spin polarization either increases or decreases.
![]() 67 degrees: the margin angle between applied magnetic field and spin direction of existed spin-polarized conduction electron
67 degrees: the margin angle between applied magnetic field and spin direction of existed spin-polarized conduction electron
smaller than 670 ![]() Spin polarization increases
Spin polarization increases
smaller than 670 ![]() Spin polarization decreases
Spin polarization decreases
The spin torque is always accompanied by an additional spin relaxation. When existed spin-polarized electrons combine with spin-polarized electron of a different spin angle created by the magnetic field, the total number of spin-polarized electrons decreases. This decrease can be large so resulting number spin-polarized electrons becomes smaller than prior-existed number and the spin polarization decrease in the magnetic field. Or the decrease can be small so resulting number spin-polarized electrons becomes larger than prior-existed number due to newly created spin-polarized electrons and the spin polarization increase in the magnetic field. The margin angle between these two cases is 670(see calculations below)
In a magnetic field there is a precession of spins of spin-polarized electron around the magnetic field. Also, the spin direction of the spin-polarized electrons slowly aligns itself along the direction of the magnetic field. The alignment of spin direction of spin-polarized electrons along the magnetic field is joint work of precession damping and the spin torque.
In a magnetic field the spin-polarized electrons are constantly created (spin pumping). The spin direction of these created is along the magnetic field. The spin of existed spin-polarized conduction electrons precess along the magnetic field. Both groups of the spin-polarized electrons combine into one group, which spin direction becomes closer and closer to the direction of the magnetic field.
A. No. Any spin precession means that the magnetization changes its direction in time. According to the Maxwell's equations, any change of magnetization generates an electromagnetic wave (See here). The emission of photons removes both the energy and spin from the electron gas. Therefore, the electron gas is not in an equilibrium. The spin precession may exist in an equilibrium only if there is an additional source of the energy and the spin, which compensates the energy and spin loss due to the magnetization precession. Note, the emission of photons is one of origins of precession damping (See here).
 Illumination by circularly polarized light, which is spin-polarized. The transfer of the photon spin to an electron gas
Illumination by circularly polarized light, which is spin-polarized. The transfer of the photon spin to an electron gas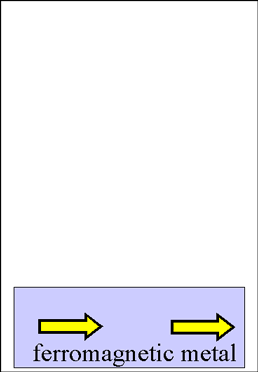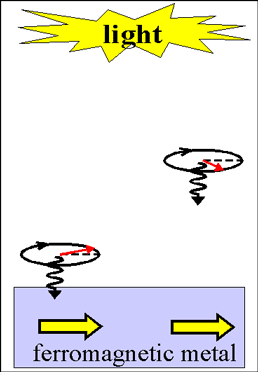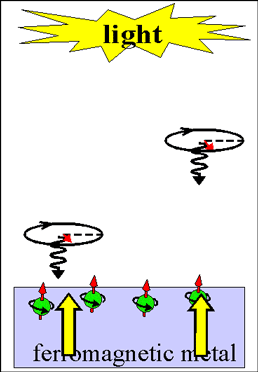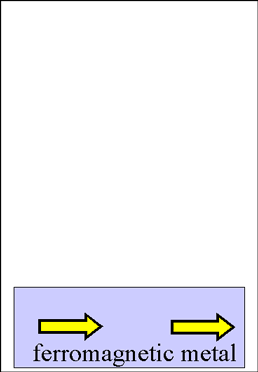 |
Fig.2 Animated picture. Changing the magnetization direction of a ferromagnetic metal by circularly polarized light. Without light illumination the magnetization of ferromagnetic film is in the plane (yellow arrows). Circularly polarized light creates the spin-polarized electrons with spin direction along light incident direction (normal to the film) The green balls with arrows show the light-created spin-polarized electrons. The spin direction of existed spin-polarized electrons is along magnetization (yellow arrows). The interaction of existed and light-created spin-polarized electrons induces a spin torque, which rotates the spin direction of existed spin-polarized conduction electrons away from the magnetization direction. Because of the exchange interaction between conduction and d- electrons, the magnetization rotates following the rotation of the spin direction of the conduction electrons. After the illumination has stopped, the magnetization returns to in-plane. |
When absorbed, a circularly- polarized photon may convert a conduction electron from the group of spin-unpolarized electrons or from group of spin-inactive electrons to the group of spin-polarized electrons (See here about spin distributions in different groups of electrons). The spin of circularly- polarized photon is one (See here) and it transformed to the electron gas when a photon is absorbed by a conduction electron. The spin direction of the optically-created spin-polarized electrons is along the incident direction of light.
In the case when the electron gas is already spin-polarized (e.g. in a ferromagnetic metal), the spin-direction of photo-excited spin-polarized electrons may not coincide with the spin direction of already-existed spin-polarized electrons. In this case, light induces the spin torque, which turns the spin direction of the existed spin-polarized electrons toward the incident direction of light.
Circularly- polarized light may change the magnetization direction in a ferromagnetic metal. For example, when circular polarized light incidents normally on a thin film of a soft ferromagnetic metal (See Fig.2), which magnetization is in plane, it induces a spin torque. The spin torque rotates the spin direction of the spin-polarized conduction electrons from the in-plane direction to the normal-to-plane direction. Since the spin direction of the conduction electrons is rotated away from the spin direction of the localized d-electrons, the d-electrons experience a torque due to the sp-d exchange interaction and their spins are rotated following the spin rotation of the conduction electrons.
There are several possible physical origins of the all-optical magnetization reversal. The mechanism of reversal depends on a material where it occurs:
Light interacts with delocalized conduction electrons. Light transfer its spin and creates the spin-polarized conduction electrons. These electrons creates the spin torque and the spin direction of the spin-polarized electrons turns away from its equilibrium direction. Due to the sp-d exchange interaction and scattering the spin direction of the localized d-electrons follows the turn of the spin of conduction electrons
![]() Two steps of spin rotation by light in a ferromagnetic metal
Two steps of spin rotation by light in a ferromagnetic metal![]() :
:
(step 1) Turn of spin direction of conduction electrons
Circularly-polarized light excites spin-polarized electrons in the electron gas. Spin direction of the light-excited spin-polarized electrons is along light propagation direction and this spin direction is not necessarily the same as the spin direction of already existed spin-polarized electrons. Since the spin-polarized electrons of two different spin directions cannot coexist at the same place, all spin-polarized electrons quickly (nearly immediately) combine into one group of the spin-polarized electrons. The spin direction of this combined group of the spin-polarized electrons is between the spin directions of existed and light-excited spin-polarized electrons. As a result, the spin polarization of the spin-polarized turns away from its equilibrium direction toward the spin-direction of photo-excited electrons.
(step 2) Turn of spin direction of localized d-electrons
The sp-d exchange interaction and scatterings between delocalized conduction electrons and localized d-electrons aligns spins of both types electrons in one same direction. When spin of conduction electrons turn out from the its equilibrium direction and the direction the localized d- electrons, the spin of localized d-electrons follows the rotation and turns to be along the spin of the conduction electrons.
A. Light interacts more effectively with conduction electrons, because their larger size.
Interaction between two particles are most effective when they have the same size. The size of a photon is about 0.1 mm -1 mm (from a light bulb) and 1 mm -1 m (from a laser). The size an electron is substantially smaller. The length of a conduction electrons is called the mean-free path λ mean. The size of a conduction electrons ( λ mean) is about 30-200 nm in a semiconductor and 0.5~20 nm in a metal. The size of a localized d-electron is about the size of atomic orbital ~0.1 nm. As a result, the interaction of a photon with a conduction electron is more effective than with a localized electron.
Light excites the electrons, which are responsible for the super-exchange interaction or the exchange interaction, to a higher energy level. The properties (spin, orbit and so forth) of excited electrons are different from the properties they had at unexcited state. It breaks the equilibrium spin arrangement.
mechanism: Light excites an electron responsible for the super-exchange (exchange) interaction on a higher energy level. This locally reduces or turns off the super-exchange (exchange) interaction between them and the magnetization start to precess or it may even be reversed.
The case is calculated when there are two groups of spin-polarized electrons with different spin directions. It is calculated how these two groups are mixed up by scatterings to combine into a single group of the spin-polarized electrons of a single spin direction.
![]() (calculation 1) The final number of spin-polarized electrons and their direction is calculated from the conservation law of the time-reversal symmetry and the total spin of the electron gas.
(calculation 1) The final number of spin-polarized electrons and their direction is calculated from the conservation law of the time-reversal symmetry and the total spin of the electron gas.
The spin is a quantum-mechanical object. The spins cannot be simply integrated or sum up. The quantum-mechanical rules have to be used. It is very difficult to do in the case of many electrons with different directions of spins. Additional difficulties is that the spin direction of each electron may be changed after a scattering (See here). The conservation law of the time-reversal symmetry greatly helps such calculations. In an equilibrium, there is only one group of spin-polarized electrons of the same spin direction. Additionally there are spin-unpolarized and spin-inactive electrons (See here). In the case when the electron gas does interact with any external "spin" objects (close system), the electron scatterings do not change the total spin of the electron gas and degree of its time- inverse symmetry. Such scatterings are called spin-independent scatterings(See here). Since the time-inverse symmetry does not change during mixing of two groups of spin-polarized electrons by scatterings, the final spin direction and spin polarization is evaluated from comparison of initial degree of time- inverse symmetry of two groups of spin-polarized electrons with final degree time- inverse symmetry of one group of spin-polarized electrons.
![]() (calculation 2) Tracing of the evaluation of spin distribution in time
(calculation 2) Tracing of the evaluation of spin distribution in time
In this case, the change of spin distribution is calculated after each scattering event. The calculation traces step by step evolution of spin distribution from initial state, which is the sum of two distributions with different spin angles, to the final distribution of a single spin angle. The spin properties of all possible scatterings between spin-polarized, spin
| interaction of two groups of spin-polarized electrons of different spin directions | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
| Volume of the ball is proportional to the number of spin-polarized electrons in each group. Arrow shows the direction of spin polarization for each group. Broken parts show spin-unpolarized electrons. | |||||||||
| The number of spin-polarized electron in both groups is the same. | |||||||||
| click on image to enlarge it |
![]() (simple case 1):
(simple case 1): Two groups of spin-polarized electrons with the same number of electrons
Two groups of spin-polarized electrons with the same number of electrons![]()
resulting spin angle: exactly between initial two angles

resulting number of spin-polarized and spin-unpolarized electrons:
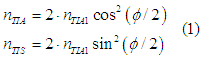
where nTIA1 is the number of spin-polarized electrons in each initial group of before interaction and phi is the initial angle between spin directions of two groups of spin-polarized electrons.
![]() (simple case 2):
(simple case 2): Number of electrons in one group spin-polarized electrons much smaller than in other group
Number of electrons in one group spin-polarized electrons much smaller than in other group![]()
resulting spin angle: spin angle of the larger group slightly rotates
![]() (general case):
(general case): Two groups of spin-polarized electrons with different numbers of electrons
Two groups of spin-polarized electrons with different numbers of electrons![]()
In the case of the interaction of two groups of different numbers of spin-polarized electrons, it takes several scattering events 5 until they relax into one group of one spin direction. For example, let us consider the groups named TIA1 and TIA2 when the number of spin-polarized electrons in group TIA1 is greater than the number of spin-polarized electrons in group TIA2.
![]()
The spin direction of TIA1 is along the z-axis and the spin direction of TIA2 has angle φ with respect to the z-axis.
After the first scattering event 5 the spin direction of TIA2 group will have the angle φ/2 with respect to the z-axis, the spin direction of TIA1 will not change. The number of spin-polarized electrons nTIA1 , nTIA2 in TIA1 and TIA2 groups and number of spin-unpolarized electrons nTIS after the first scatterings are calculated as (See scattering event 5):
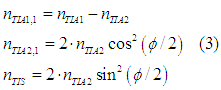
After the second scattering event 5, the angle between the spin directions of two groups of spin-polarized electrons will be φ/4 and the numbers of spin-polarized and spin-unpolarized electrons can be calculated as
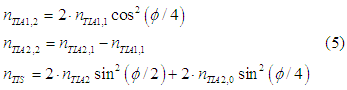
where the number of spin-polarized electrons in the group TIA2 is assumed to be larger than in group TIA1: nTIA2> nTIA1
Therefore, after each scattering event 5, the spin angle between the two groups of spin-polarized electrons is reduced by a factor of 2. The spin direction of the group, in which there were fewer spin-polarized electrons, rotates and the number of electrons in this group increases. The spin direction of the group, in which there were more spin-polarized electrons, does not rotate and the number of electrons in this assembly decreases.
![]() The most of the spin-polarized electrons make one group of the same spin direction very quickly (See the numerical simulation below).
The most of the spin-polarized electrons make one group of the same spin direction very quickly (See the numerical simulation below).
It should be noted that the probability of scattering event 5 is not constant, but it is proportional to the number of spin-polarized electrons in the TIA1 and TIA2 groups. The probability pscat5 of one scattering event 5 in the electron gas can be calculated as (See scattering event 5 )

where nTIA1 , nTIA2 are numbers of spin-polarized electrons in TIA1 and TIA2 groups, nTIS is the number of spin-unpolarized electrons and pscat5,0is the probability of a single scattering event 5.
![]() The most of the spin
The most of the spin
|
||
Fig 1. The interaction of two groups of spin-polarized electrons. (left): Animated polar figure. The radius of arrows corresponds to the probability of electrons to be in one of groups of the spin polarized electrons and the angle indicates the spin direction of each group. The animated parameter is the number of scattering events 5. (right): Occupation probabilities of TIA1 (black line) and TIA2 (red line) groups of spin-polarized electrons as a function of number of the number of scattering events 5. Green line shows the probability for the total number of spin-polarized electrons (TIA=TIA1+TIA2). The yellow line show the probability for the spin-unpolarized electrons (TIS). (Inset) Inset shows spin directions of electrons of TIA1 and TIA2 groups. |
||
| Initial conditions: Occupation probability is 0.8 for TIA1 and 0.2 for TIA2. It means that 80 % of spin-polarized electrons are in group TIA1 and their spin angle is 0 deg.. 20 % of spin-polarized electrons are in group TIA2 and their spin angle is 145 deg.. There is no spin-unpolarized electrons. |
Initial conditions: Figure 1 shows the calculated interaction of two groups TIA1 and TIA2 of spin-polarized electron when initially all electrons are spin-polarized (sp=100 %), 80 % of the spin-polarized electrons belongs to group TIA1 (80 % of electrons have the same spin direction), 20 % of the spin-polarized electrons belongs to group TIA1 (20 % of electrons have a different spin direction from electrons of TIA1 group), and there are no spin-unpolarized electrons. The initial angle between the spin directions of two groups is 145 deg. Figure 2 shows a similar case of the interaction of the TIA1 and TIA2 groups of the spin-polarized electrons . However, initially 98 % of the spin-polarized electrons are in the TIA1 group and only 2 % of the spin-polarized electrons are in the TIA2 group. Similarly, the initial angle between the spin directions of two groups is 145 deg.
Number of spin-polarized electrons in each group: After each scattering event 5 the angle between the spin directions of two groups of spin-polarized electrons decreases and some electrons become spin-unpolarized. During the first 3-4 scattering events 5 , a significant number of spin-polarized electrons becomes spin-unpolarized (there is a significant spin relaxation). Additionally, the number of spin-polarized electrons in the TIA1 group decreases and the number of spin-polarized electrons in the TIA2 group increases until the numbers of spin-polarized electrons of both spin angles become nearly equal. After that, the numbers oscillate approaching the same number.
Spin direction: During a few first scatterings, the spin direction of the spin-polarized electrons of TIA2 group (a smaller number of electrons) changes substantially. In contrast, the change of spin direction of the spin-polarized electrons of TIA1 group (a larger number of electrons) is very small (nearly no change). The change becomes larger and substantial after 5-7 scatterings, when the numbers of spin-polarized electrons in both groups of different spin angles become comparable.
|
||
Fig 2. The interaction of two group of spin-polarized electrons of different spin angles. This figure is similar to Fig 1, but the initial conditions are different: There are 10 times smaller electrons in group TIA2 |
||
| Initial conditions: 98 % of spin-polarized electrons are in group TIA1 and their spin angle is 0 deg.. 2 % of spin-polarized electrons are in group TIA2 and their spin angle is 145 deg.. There is no spin-unpolarized electrons. |
![]() Interaction time: After 10-15 scatterings, the angle between the spin directions of spin- polarized electrons of two groups becomes very small and the two groups may be considered as one group of spin-polarized electrons of one spin direction..
Interaction time: After 10-15 scatterings, the angle between the spin directions of spin- polarized electrons of two groups becomes very small and the two groups may be considered as one group of spin-polarized electrons of one spin direction..
|
||
Fig P11. The interaction of two group of spin-polarized electrons of different spin angles. This figure is similar to Fig 1, but the initial conditions are different: The angle between spins of two groups of spin-polarized electrons is 179 deg.. Theretofore, spin directions is nearly opposite. |
||
| Initial conditions: 80 % of spin-polarized electrons are in group TIA1 and their spin angle is 0 deg.. 20 % of spin-polarized electrons are in group TIA2 and their spin angle is 175 deg.. There is no spin-unpolarized electrons. |
When the initial spin directions of two group of spin polarized electrons are opposite, the resulting remaining number of spin-polarized electrons is difference between spin-polarized electrons of two groups. The resulting spin-angle is the along the spin direction of the group of the larger amount of electrons.
Figure P11 shows the case when the angle between spin directions of two is 179 degrees. It is near opposite.
In this case, the spin-polarized electrons of group TIA2 become spin-unpolarized very quickly. Even the most of the electrons of the TIA2 group are converted into spin-unpolarized already after 1st scattering, a tiny amount of electrons still remains in TIA2 group. Afterwards, after each scattering the electron amount in this group sharply increases. After 12 scatterings, the amount of electrons in the TIA2 group becomes comparable with the amount of electrons in the TIA1 group.
| Dependence on a relative amounts of electrons in each group | ||
|---|---|---|
|
||
Fig.3 The final amounts of spin-polarized and spin-unpolarized electrons after interaction of two groups of spin-polarized electrons of different spin angles as a function of a relative amounts of electrons in group TIA2. (left) final spin angle of spin-polarized electron with respect to the initial spin angle of spin-polarized electrons of TIA1 group. (right) final amounts of spin-polarized and spin-unpolarized electrons. |
||
| Initial conditions: The angle between spins of electrons of TIA1 and TIA2 groups is 145 deg. . There is no spin-unpolarized electrons. Click on image to enlarge it |
Figure 3 shows the rotation angle and the amount of electrons converted from both groups of spin-polarized electrons to the group of spin-unpolarized electrons as a function of related amounts of spin polarized electrons in each group. The probability of 0.5 means that there is an equal amount of electrons in each group.
Figure 4 shows the rotation angle and the amount of electrons converted from both groups of spin-polarized electrons to the group of spin-unpolarized electrons as a function of the angle between spin directions of electrons of two groups.
no spin rotation: There is no spin rotation in the cases when the spin direction of spin-polarized electrons of group TIA1 and TIA2 are parallel or antiparallel of spin direction of electrons of the group TIA2.
| Dependence on angle between spins of two groups | ||
|---|---|---|
|
||
| Fig.4 The interaction of two groups of spin polarized electrons as a function of angle φ between the spin directions of injected (TIA2) and existed (TIA1) spin-polarized electrons .(left) final spin angle of spin-polarized electron with respect to the initial spin angle of spin-polarized electrons of TIA1 group. (right) final amounts of spin-polarized and spin-unpolarized electrons. | ||
| Initial conditions: The 99 % of spin-polarized electrons were in the group TIA1 and 1 % of spin-polarized electrons were in group TIA2. There is no spin-unpolarized electrons. Click on image to enlarge it |
It always decreases comparing to the total amount of initial spin-polarized electrons (TIA1+TIA2).
The final amount of spin-polarized electrons may either decrease or increase comparing to the initial amount in the larger group (TIA1). It depends on the spin directions in two groups.
The number of spin-polarized electrons, which become spin-polarized, increases when the angle between the spin directions of electrons of two groups increases. When the angle is 120 degrees and larger, the number of converted spin-unpolarized electrons is about 2%. This is twice the initial amount of spin- polarized electrons in the group TIA2. For angles smaller than 67 degrees, the amount of converted spin-unpolarized electrons is smaller than the initial amount of spin-polarized electrons in the group TIA2.
For angles smaller than 67 degrees, the final number of spin-polarized electrons is larger than the initial number in the group TIA1 (the group of the larger initial number of electrons). Therefore, we may say that the number of spin-polarized electrons in the TIA1 increases as a result of interaction with group of TIA2. In contrast, in the case of angles greater than 67 degrees, the number of spin-polarized electrons in the group TIA1 decreases due to the interaction.
The spin torque is calculated from the condition of an equilibrium of injected rate of spin-polarized electrons (TIA2) and the conversion rate of spin-polarized electrons from group TIA2 into group TIA1 of the existed spin-polarized electrons and the group of spin-unpolarized electrons (See Eq.(25)). Even though the calculations are done numerically using the method of calculations of Fig.1 and Fig.2 , the results are expressed analytically with calculated parameters A(φ) and R(φ), which are defined as the spin torque coefficient and the spin-relaxation coefficient. The dependence of the spin torque on different material and injection parameters can be calculated from calculated dependencies of A(φ) and R(φ) on those parameters.
In the case when a small amount of spin-polarized electrons of the group TIA2 is continuously injected at a rate![]() into the region where there are spin-polarized electrons of the TIA1 group, the spin-polarized electrons of TIA1 group experience a spin torque, which can be calculated as as
into the region where there are spin-polarized electrons of the TIA1 group, the spin-polarized electrons of TIA1 group experience a spin torque, which can be calculated as as
where nTIA1 is the number of spin-polarized electrons in the group TIA1, ![]() are unit vectors directed along spin directions of electrons of the TIA1 and TIA2 groups,, respectively, and A(φ) is the spin torque coefficient, which depends on the angle φ between the spin directions of injected and existed electrons.
are unit vectors directed along spin directions of electrons of the TIA1 and TIA2 groups,, respectively, and A(φ) is the spin torque coefficient, which depends on the angle φ between the spin directions of injected and existed electrons.
The conversion rate (spin relaxation rate) of spin-polarized electrons into spin-unpolarized can be calculated as
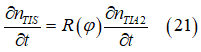
where R(φ) is the spin-relaxation coefficient, which depends on the angle φ between the spin directions of injected and existed electrons.
old form: ![]()
|
||
Fig.5 (left) Spin torque coefficient A(φ) (See Eq. (20)) and (right) spin-relaxation coefficient R(φ) (See Eq. (21)) as a function of angle φ between the spin directions of injected (TIA2) and existed (TIA1) spin-polarized electrons. 1 % of spin-polarized electrons in TIA2 group (injected) , 99% of spin-polarized electrons in TIA1 group (existed). Click on image to enlarge it |
Figure 5 shows the spin torque coefficient A(φ) and the spin-relaxation coefficient B(φ) (TIS conversion coefficient) as a function of angle φ between the spin directions of injected and existed electrons (angle between spins of TIA1 and TIA2 groups).
Note: For small angles φ (φ<30 deg) , A(φ) ~57 deg and R(φ) ~0.
Figure 6 shows the spin torque coefficient A(φ) and the spin-relaxation coefficient B(φ) (TIS conversion coefficient) as a function of the amount of injected spin-polarized electrons (TIA2) with respect to the number of existed spin-polarized electrons (TIA1) for different angles φ . The number is scanned from 0 to 40%. Note: the value of the coefficient B(φ) is between 1 and 2 (the variation is small).

|
||
Fig.6 (left) Spin torque coefficient A(φ) (See Eq. (20)) and (right) spin-relaxation coefficient R(φ) (See Eq. (21)) as a function of the amount of injected spin-polarized electrons (TIA2) with respect to the number of existed spin-polarized electrons (TIA1). Click on image to enlarge it |
![]() the spin torque is linearly proportional to the injection rate .
the spin torque is linearly proportional to the injection rate .
![]() As was calculated in Fig.1 and Fig.2, the interaction of two groups of spin-polarized electrons with different spin angles takes some time until they are fully transformed into one group of spin-polarized electrons of the same spin direction. We assign t2 as the effective time, after which the spin direction of electrons of the TIA1 group is fully rotated into its final direction. Even though a tiny oscillations around the final angle persists for a long time, the defined time t2 is the time, after which the deviation of the spin angle from its final position is less than 0.1 degrees. For examples of Fig.1 and Fig.2, , the time t2 corresponds to a time duration of ~5-7 scattering events 5. The number of spin-polarized electrons ( TIA2), which are injected during the time t2, is calculated as
As was calculated in Fig.1 and Fig.2, the interaction of two groups of spin-polarized electrons with different spin angles takes some time until they are fully transformed into one group of spin-polarized electrons of the same spin direction. We assign t2 as the effective time, after which the spin direction of electrons of the TIA1 group is fully rotated into its final direction. Even though a tiny oscillations around the final angle persists for a long time, the defined time t2 is the time, after which the deviation of the spin angle from its final position is less than 0.1 degrees. For examples of Fig.1 and Fig.2, , the time t2 corresponds to a time duration of ~5-7 scattering events 5. The number of spin-polarized electrons ( TIA2), which are injected during the time t2, is calculated as
where ![]() is the injection rate.
is the injection rate.
Under a continuous and constant injection rate, ΔnTIA2 is the constant number of spin-polarized electrons of the group TIA2, which spin is not yet aligned along spin direction of the existed spin-polarized electrons of group TIA1. These spin-polarized electrons of the group TIA2 are in the process of the conversion to the group TIA2 and to the group of spin-unpolarized electrons. As sooner as they are converted, the same number of spin-polarized electrons TIA2 is injected. As a result, ΔnTIA2 remains a constant.
When the ΔnTIA2 exceeds 1 % of the number of spin-polarized electrons in the group TIA1 (the number of the existed spin-polarized electrons), the injected rate is defined as fast and the dependence of the coefficients A(φ) and B(φ) on the injection rate should be taken into calculations.
| Spin Torque. Two spin pumps |
|---|
| When only one spin pump is on, the spin direction of electron gas (big ball) towards the spin pump direction. When both spin pumps are switched on, the the spin direction of electron gas is between spin directions of the spin pumps. |
 |
| Big ball shows a large number of spin-polarized electrons of electron gas. The small balls shows direction of injected spin-polarized electrons from two spin pumps. |
| Spin direction of the front spin pump is toward left. Spin direction of the backside spin pump is toward up. |
| arrows shows the spin-direction and the volume of balls is proportional to the number of the spin polarized electrons |
| click on image to enlarge it |
A. No. The electrons are frequently scattered between different groups. It is only average number of electrons in each group remains unchanged after a frequent scatterings (See here). It contrasts to the case of "holes" and "electrons" in a semiconductor. The electron scattering between a "hole" and "electron" is a very rare event, because of their different spacial symmetry (See here) and an electron stays as a "hole" and "electron" for a long time in a semiconductor. (Note, the case of a metal is different)
Spin-dependent photo- luminescence: Spin-polarized electrons are excited by a circular- polarized light. When these electrons relax to the ground energy level, the photo-luminescence light is emitted.
The Hanle effect describes the fact that the degree of circular polarization of the Spin-dependent photo- luminescence decreases when a magnetic field is applied perpendicular to the spin-polarization
![]() How the external perpendicular magnetic field affect the spin-polarized electrons?
How the external perpendicular magnetic field affect the spin-polarized electrons?
(1) There is a spin precession around the external magnetic field
(2) the spin aligned towards the external magnetic field
 or here
or here
I will try to answer your questions as soon as possible